Haem- Bone Marrow
The technologist’s task in bone marrow aspiration procedures is to carry out on-site smearing of patient’s marrow. Usually there are 3 different types of smears done:
1) Direct aspirate/ wedge smears:
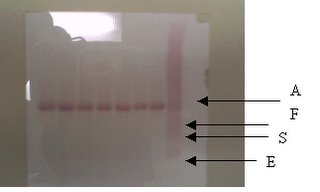
-Performed by placing a few marrow particles at one end of a clean slide and smearing with a spreader.
-Marrow particles (fragments) must be found mostly at the tail of the smear.
-After staining slides with MayGrunwald Giemsa stain, the fragments appear bluish in colour.
-When viewing under a microscope, a trail of cells can be seen following the fragments. Differential counts are performed in these cellular trails.
-Fragments are examined to determine the cellularity.
Wedge Smear
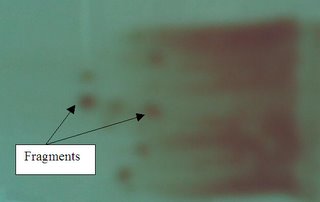
2) Squashed Preparation
-A few particles (fragments) are firstly placed on a slide. Another slide is then gently compressed on the first slide to spread and disperse the particles.
-The crushed fragments will be at the center of the smear.
-To see the cells of the fragments more clearly. (since the fragments are more spread out when it is squashed)
3) Trephine Imprint (from bone)
-Several (2-4 slides) touch or imprint preparations were made by gently touching the core plug (bone) and rolling it between 2 slides.
-This smear or preparation is especially useful if the aspirated marrow is diluted or a “dry tap” (i.e. no marrow particles or fragments can be extracted) is obtained.
-When ‘rubbing’ the core plug between slides, the cells on the surface of the core plug will be attached to the slide.
Other possible presence of cells to take note or when viewing marrow slides:
-Nucleated red blood cells (nRBCs)
-Megakaryocytes
-Mitotic cells (undergoing division)
Confirmatory tests in relation to possible diagnosis:
1) Iron stain (demonstrate presence of hemosiderin/storage iron in erythroblasts/sideroblasts or erythrocytes): useful in the classification of anemia associated with defective Hb synthesis. Can also be used to identify presence of ringed sideroblasts in the diagnosis of soderoblastic anemia and myelodysplasia.
2)Neutrophil Alkaline Phosphatase (NAP score): Used nto distinguish chronic granulocytic leukemia (CGL) from other myeloproliferative disorders and Polycythaemia Rubra Vera from secondary polycythaemia.
3)CSF test for blast cells: used to examine leukaemic cells in the CSF from a cytospin preparatrion. Essential in the diagnosis of meningeal leukaemia. Also used to monitor the adequacy and effectiveness of treatment.
4) Periodic Acid Schiff (PAS) reaction : stains a variety of carbohydrates including glycogen (found in haemopoietic cells). Used in differential diagnosis of acute leukaemia (e.g: Lymphoblasts are PAS positive.